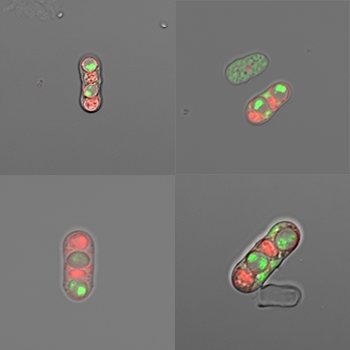
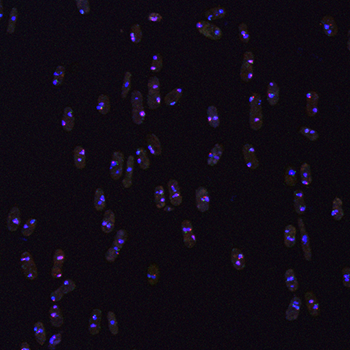
Our laboratory uses an evolution-guided molecular approach to understand the causes of infertility. In particular, we are interested in the effects of genetic conflicts caused by selfish components embedded in eukaryotic genomes.
These parasitic components include transposable elements, which can proliferate by copying and pasting themselves into new regions of the genome, as well as meiotic drive alleles. Meiotic drive alleles persist and spread in populations by biasing their transmission into functional gametes (reproductive cell such as sperm). For example, a meiotic drive allele on a male’s (XY) X chromosome could drive the male to father exclusively daughters by generating only X-bearing sperm. Transposons and meiotic drive alleles can cause infertility, but the molecular mechanisms by which they act in meiosis are not well understood. Our research proposes that conflicts driven by these parasites are major drivers of genome evolution.
The current focus of my lab’s research is on a class of meiotic drive alleles discovered in fission yeasts. These drive alleles act in heterozygotes (driver+/driver-) to kill the (driver-) gametes that fail to inherit the selfish allele. The drive alleles have no apparent redeeming features: their sole function appears to be to selfishly destroy their competition. This selfish behavior comes at a significant cost to fertility. We have discovered many independently acting meiotic drive alleles that cause the very rapid evolution of reproductive isolation, that is speciation between very closely related populations that are 99.5% identical at the DNA sequence level. We are using this very simple model system to understand the molecular mechanisms by which gametes can destroy each other. In addition, we are testing how these selfish alleles can gain a foothold and expand within a genome in spite of the fact that they cause infertility.