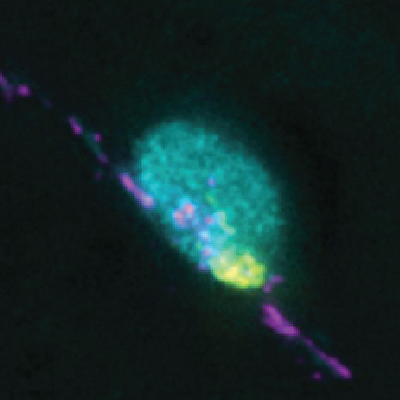
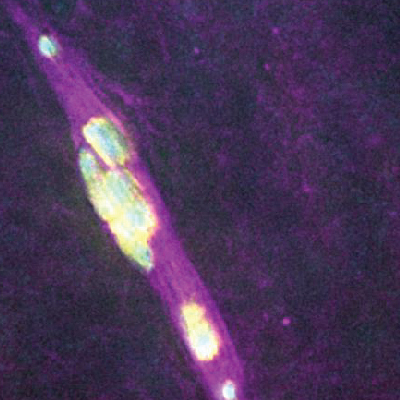
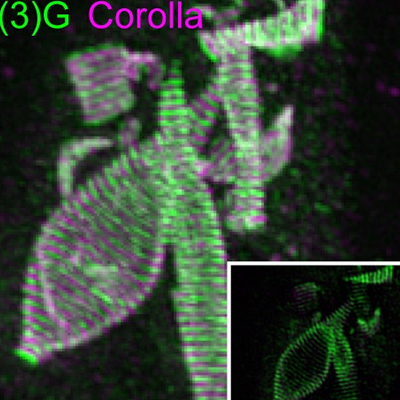
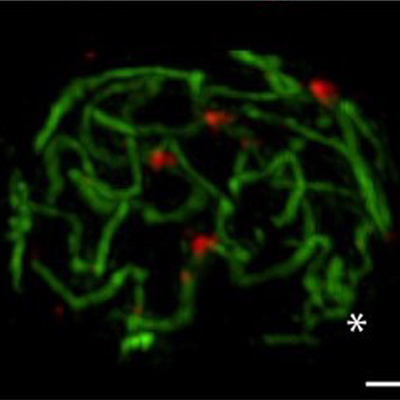
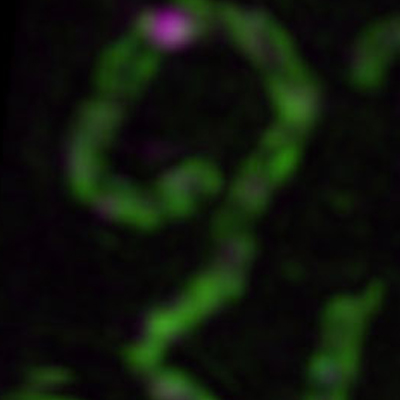
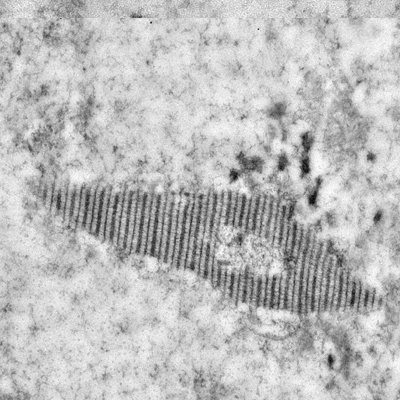
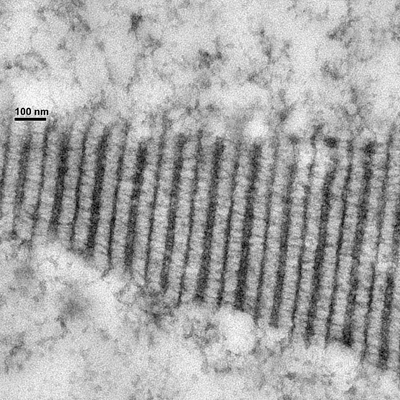
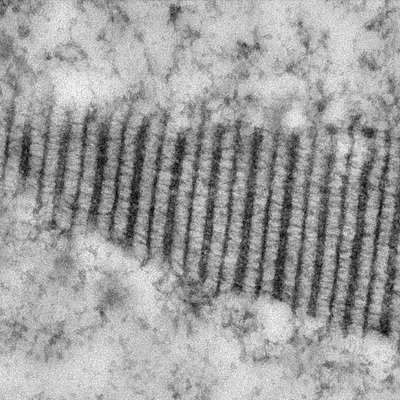
- how chromosomes can find their homolog during meiosis,
- the structure and function of the giant protein structure that connects them known as the synaptonemal complex, and
- how paired homologs can segregate from their partners.
Research
Chromosome synapsis, recombination, & segregation
We are highly focused on understanding the process of pairing, with a focus on understanding the roles of centromeres and heterochromatin in mediating this process. We are also intent on elucidating the structure and function of the SC and the various roles that it plays in meiosis. Although much is known about the structure and the composition of the SC, critical questions remain regarding its function and the detailed topology of its components.
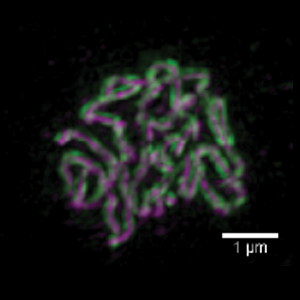
Why we care so much about this problem: From a basic research perspective we want to understand meiosis because it is the physical basis of Mendel’s Laws. We also know that a knowledge of meiosis will answer the question of how two thing pair. But, in a broader sense, we are aware that improper meiosis is a common cause of miscarriage, impaired fertility, and birth defects in human beings. We know, for example, that the risk of certain kinds of meiotic failures (the generation of trisomies) greatly increases with advancing maternal age. The ability to address these issues, and perhaps someday ameliorate them, will come only when we know what goes “wrong” to cause them – and that can come only when we fully understand how meiosis actually works.
Lab members

Scott Hawley, Ph.D.
-Investigator and American Cancer Society Research Professor
-Dean Emeritus, The Graduate School of the Stowers Institute
-Professor of Molecular and Integrative Physiology School of Medicine, University of Kansas Medical Center
-Adjunct Professor, School of Biological Science, University of Missouri at Kansas City
I need to do both to live."
Professional Societies
2001
Fellow, American Association for the Advancement of Science
2006
American Academy of Arts and Sciences
2010
President, Genetics Society of America
2011
National Academy of Sciences
2018-Present
Chair, NIH NICHD Developmental Biology Subcommittee
Honors
1984
Searle Scholar
2008
Excellence in Education Award - Genetics Society of America
2013
George W. Beadle Award Genetics Society of America
2015
American Cancer Society Excellence in Research Award
Education
B.S., Biology
University of California, Riverside
Ph.D., Genetics
University of Washington
Recent Publications
Selected Research Papers
Highly contiguous assemblies of 101 drosophilid genomes
Kim BY, Wang JR, Miller DE, Barmina O, Delaney E, Thompson A, Comeault AA, Peede D, D'Agostino ERR, Pelaez J, Aguilar JM, Haji D, Matsunaga T, Armstrong EE, Zych M, Ogawa Y, Stamenković-Radak M, Jelić M, Veselinović MS, Tanasković M, Erić P, Gao JJ, Katoh TK, Toda MJ, Watabe H, Watada M, Davis JS, Moyle LC, Manoli G, Bertolini E, Košťál V, Hawley RS, Takahashi A, Jones CD, Price DK, Whiteman N, Kopp A, Matute DR, Petrov DA. Elife. 2021 Jul 19;10:e66405. doi: 10.7554/eLife.66405.
Regulation of Polo Kinase by Matrimony Is Required for Cohesin Maintenance during Drosophila melanogaster Female Meiosis
Curr Biol. 2020.
X chromosome and autosomal recombination are differentially sensitive to disruptions in SC maintenance
Billmyre KK, Cahoon CK, Heenan GM, Wesley ER, Yu Z, Unruh JR, Takeo S, Hawley RS. Proc Natl Acad Sci U S A. 2019;116:21641-21650.
The E3 ubiquitin ligase SINA regulates the assembly and disassembly of the synaptonemal complex in Drosophila females
Hughes SE, Hemenway E, Guo F, Yi K, Yu Z, Hawley RS. PLoS Genet. 2019;15:e1008161. doi: 10.1371/journal.pgen.1008161.
Narya, a RING finger domain-containing protein, is required for both meiotic DNA double-strand break formation and crossover maturation in Drosophila melanogaster
Lake CM, Nielsen RJ, Bonner AM, Eche S, White-Brown S, McKim KS, Hawley RS. PLoS Genet. 2019;15:e1007886. doi: 1007810.1001371/journal.pgen.1007886.
Reviews, Commentaries, Chapters or Books
Synaptonemal complex
Lake CM, Hawley RS. Curr Biol. 2021;31(5):R225-R227. doi:10.1016/j.cub.2021.01.015
Alternative Synaptonemal Complex Structures: Too Much of a Good Thing?
[published ahead of print August 13 2020]. Trends Genet. 2020.
Meiosis: Location, Location, Location, How Crossovers Ensure Segregation
Curr Biol. 2020
The Human Genome, A User's Guide.
Third ed: Academic Press; 2011.
Drosophila: A Laboratory Handbook
2nd ed. New York: Cold Spring Harbor Press, 2005. 1409 p.
News

Former Undergraduate Research, Nazanin Yeganeh Kazemi, receives the Paul & Daisy Soros Fellowship for New Americans.
Read more
Former Undergraduate Researcher, Emily Wesley, represents UMKC at Undergraduate Research Day at the Capitol (URD@C)
Read more
Virtual Presentation Link:
https://event.crowdcompass.com/ugrd2021/activity/buTdA76CMP

"Identification and Characterization of Breakpoints and Mutations on Drosophila melanogaster Balancer Chromosomes" earns G3 Spotlight.
Read more”Everyone in the Hawley Lab congratulates Camilla and wishes her well!

https://academic.oup.com/g3journal/pages/spotlight
Undergrad research
Want to be an undergraduate intern in the Hawley lab?
Featured Undergrads

Emily Wesley, UMKC Undergraduate
Press
Pre-Med Biology Student Publishes Article in Scientific Journal

Elizabeth Hemenway

Danny Miller
Danny Miller currently is a combined pediatrics and medical genetics resident at Seattle Children’s Hospital and the University of Washington. Clinically, he is interested in unsolved genetic disorders and how long-read sequencing might be used to both increase the rate of genetic diagnoses and decrease the amount of time it takes to make a genetic diagnosis

Nicole Nuckolls
Working with Scott Hawley was a dream come true for Nicole. She has noted that she would not be the scientist she is today without the experience in his lab. She still uses genetics as her primary tool of research and is still studying the principles of cell division and meiosis, now as a postdoc. She is currently working as a postdoc at the University of Colorado in Julia Cooper's lab, studying fission yeast telomeres and centromeres. The training she received in the Hawley lab, not only in scientific method and experimental procedures, such as microscopy and genetics, but generally as a scientist, have lasted her throughout her studies. She notes these skills and preparatory learning will continue to guide her in her future career. Scott taught her to think critically and how to mentor the future generations of scientists (and truly enjoy it), while instilling a love of meiosis, genetics, and science, in general.

Nazanin Yeganeh Kazemi

Scott Beeler
What we're reading
A sampling of recent literature of special interest to the Hawley Lab, with occasional comments from lab members.
Hassold T, Maylor-Hagen H, Wood A, Gruhn J, Hoffmann E, Broman KW, Hunt P. Am J Hum Genet. 2021 Jan 7;108(1):16-24. doi: 10.1016/j.ajhg.2020.11.010. Epub 2020 Dec 10. PMID: 33306948.
Single-Molecule Tracking of Chromatin-Associated Proteins in the C. elegans Gonad.
von Diezmann L, Rog O. J Phys Chem B. 2021 Jun 7. doi: 10.1021/acs.jpcb.1c03040. Online ahead of print. PMID: 34097417.
Let's get physical - mechanisms of crossover interference.
von Diezmann L, Rog O. J Cell Sci. 2021 May 15;134(10):jcs255745. doi: 10.1242/jcs.255745. Epub 2021 May 26. PMID: 34037217.
Meiotic sister chromatid exchanges are rare in C. elegans.
Almanzar DE, Gordon SG, Rog O. Curr Biol. 2021 Apr 12;31(7):1499-1507.e3. doi: 10.1016/j.cub.2020.11.018. Epub 2021 Mar 18. PMID: 33740426 Free article.
Synaptonemal Complex dimerization regulates chromosome alignment and crossover patterning in meiosis.
Gordon SG, Kursel LE, Xu K, Rog O. PLoS Genet. 2021 Mar 17;17(3):e1009205. doi: 10.1371/journal.pgen.1009205. eCollection 2021 Mar. PMID: 33730019 Free PMC article.
Meiotic DNA break repair can utilize homolog-independent chromatid templates in C. elegans.
Toraason E, Horacek A, Clark C, Glover ML, Adler VL, Premkumar T, Salagean A, Cole F, Libuda DE. Curr Biol. 2021 Apr 12;31(7):1508-1514.e5. doi: 10.1016/j.cub.2021.03.008. Epub 2021 Mar 18. PMID: 33740427 Free article.
Elevated Temperatures Cause Transposon-Associated DNA Damage in C. elegans Spermatocytes .
Kurhanewicz NA, Dinwiddie D, Bush ZD, Libuda DE. Curr Biol. 2020 Dec 21;30(24):5007-5017.e4. doi: 10.1016/j.cub.2020.09.050. Epub 2020 Oct 15. PMID: 33065011 Free article.
Join us!
Interested in becoming a member of the Hawley Lab?
For motivated undergraduates, please refer to the Undergraduate Research tab for information on how to become an intern in the lab.
Students with an interest in conducting their PhD thesis work in the Hawley Lab should apply through the Graduate School of the Stowers Institute for Medical Research. Doctoral students from other PhD programs should contact Dr. Hawley directly at rsh@stowers.org
Dr. Hawley is accepting applications for independent, ambitious postdoctoral scholars on a rolling basis. To apply, please send a current CV, a brief description of your doctoral research, and a paragraph summarizing what you are most interested in working upon joining the lab.
Hawley Lab
Stowers Institute for Medical Research
1000 E 50th Street
Kansas City, Missouri 64110
KVirden@stowers.org
(816) 926-4000